SAN MARINO, Calif. — Epeius Biotechnologies announced today the promising results of an on-going United States-based Phase I/II study of Rexin-G for metastatic breast cancer that is refractory to conventional chemotherapy (J Clin Oncol 26:14509, 2008). This clinical trial employed intra-patient dose-escalations of Rexin-G given i.v. two to three times a week for 4 weeks, with doses ranging from 2 x 10e11 cfu to 6 x 10e11 cfu per week. The goal of the adaptive trial design is to confirm the over-all safety of Rexin-G and to determine the optimal dosing regimen for Rexin-G that would document the significant clinical benefits required to support a Phase II pivotal study.
The interim results of this Phase I/II study of targeted gene delivery in vivo are very encouraging-intravenous infusions of Rexin-G demonstrated significant biological activity without toxicity in patients with rapidly progressive chemo-resistant breast cancer. Once the general safety of repeated infusions of Rexin-G was documented, the FDA approved across the board intra-patient dose-escalations in order to gain better tumor control.
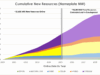
 These escalating doses of Rexin-G were associated with stabilization of disease, using both RECIST and International PET criteria, significant reductions in CA 15.3 levels, a median progression-free survival of 6 months (RECIST) and a median over-all survival of greater than 7 months with all patients surviving at the 8-month follow-up period. No dose-limiting toxicity was observed, even at the higher doses of Rexin-G, thus confirming that repeated infusions of Rexin-G are safe and well-tolerated.
These escalating doses of Rexin-G were associated with stabilization of disease, using both RECIST and International PET criteria, significant reductions in CA 15.3 levels, a median progression-free survival of 6 months (RECIST) and a median over-all survival of greater than 7 months with all patients surviving at the 8-month follow-up period. No dose-limiting toxicity was observed, even at the higher doses of Rexin-G, thus confirming that repeated infusions of Rexin-G are safe and well-tolerated.
According to Dr. Erlinda M. Gordon, Medical Director of Epeius, “The importance of these dose-escalation studies-which clearly establish safety before escalating to more potent tumoricidal levels-is a primary concern in the development of a new genetic medicine like Rexin-G.”
Taken together with the results of previous studies, the current on-going Phase I/II study confirms the exemplary safety and therapeutic potential of Rexin-G in chemotherapy-resistant metastatic breast cancer.
For more information about Rexin-G, on-going clinical trials in the USA and abroad, and/or Epeius pathotropic (disease-seeking) gene delivery systems, please contact Dr. Erlinda M. Gordon at egordon @epeiusbiotech.com.
Epeius Biotechnologies: http://www.epeiusbiotech.com.
[tags]Epeius Biotechnologies Corporation, Dr Erlinda M Gordon, chemotherapy resistant metastatic breast cancer[/tags]